- Home
- Research
- Nanobiophotonics
- Research results
- Long-Term Stability of Shape-Anisotropic Plasmonic Nanoparticles
Long-Term Stability of Shape-Anisotropic Plasmonic Nanoparticles
19.08.2019
Shape-anisotropic precious metal nanoparticles made of silver exhibit strong corrosion within hours, especially at the corners. As a result, the spectroscopic properties of the particles change significantly, making them unsuitable for optical sensor technology. Atomic force microscopy as a high-resolution method in combination with microspectroscopy allows the kinetics of this corrosion to be determined in the nanometer range.
Due to their high absorption and scattering cross section, silver nanostructures are suitable optical sensor materials. Unfortunately, the optical properties of these nanostructures are often unstable over time.
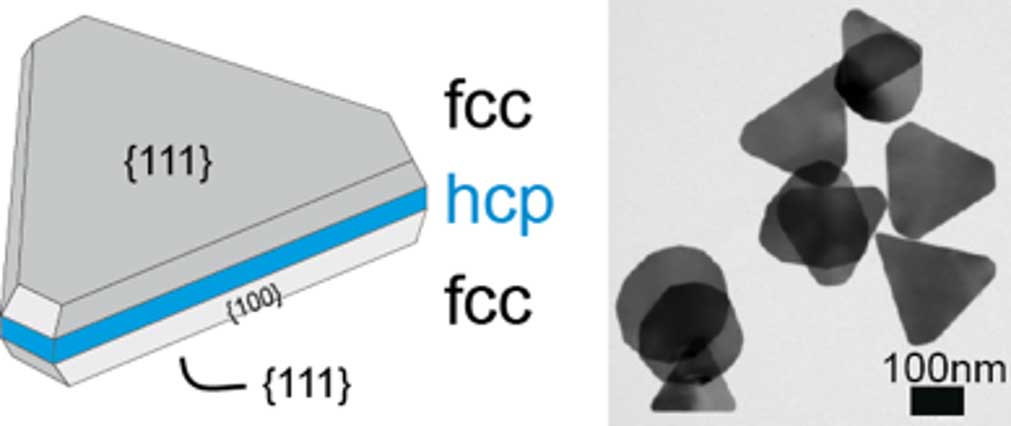
Particularly shape-anisotropic nanoparticles such as monocrystalline triangular silver nanoprisms exhibit corrosion processes (see Figure 1). This excludes the use of such particles in optical sensors as signal converters (LSPR sensors). To investigate this corrosion, silver nanoprisms were characterized by correlative, laterally high-resolution methods – such as micro-UV-VIS spectroscopy and atomic force microscopy (AFM) – under laboratory conditions at the single-particle level over a period of 24 hours.
Figure 2:
Optical (a/b) and morphological (c/e) changes of silver prisms after 24 hours. a/b: Dark-field micrograph of silver prisms prepared both freshly and after 24 hours. c/e: AFM images correlating to a and b. d: 3D-AFM image of a nanoprism prepared both freshly and after 24 hours.
After only a few hours, clear changes due to material growth were able to be seen on the tips of these particles (right side in Figure 2D). This material deposition on the corners of the triangular prisms is accompanied by a partial regression of the original prism shape (e. g. to a disk or complete degradation). To describe these changes, a corrosion parameter was introduced which describes the change over time of the maximum height compared to the average height of the prisms (CPn(t) = CP(t)/CP(0)). The AFM data shows that corrosion occurs within about 10 hours for all particles and is usually completed within one hour. The temporal beginning of this corrosion appears stochastic.
A triangular silver nanoprism consists of three crystalline layers with a surface normal parallel to the sides of the triangle. The two outer layers are formed from face-centered cubic (fcc) crystal structures with few defects (see Figure 1). The middle layer corresponds more to a hexagonal close-packed (hcp) sphere with many defects.
Apparently, corrosion starts at the defects in this hcp layer, which are only accessible at the outer edges of the particles. Accessibility is greatest at the tips, which could explain why they are the starting point of corrosion (see Figure 3). During the strong phase of corrosion, the silver atoms of the hcp layer are involved, and the deposit grows isotropically (as a sphere) with increasing volume. Growth slows down to a standstill when further silver atoms in the hcp layer can no longer diffuse efficiently to the position of the surface lattice site. A similar effect in the form of hollowed triangular particles has been observed when silver is replaced by gold in an electroplating process.
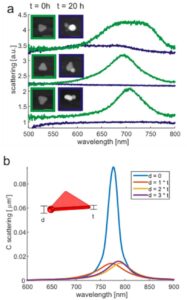
These morphological changes result in a shift in the plasmonic spectral properties. This became clear when characterizing the nanoparticles by means of microspectroscopy (see Figure 3a). The scattering spectrum of individual nanoparticles was determined before and after corrosion. Over a period of approximately 20 hours, the spectra show a clear collapse in the original particle plasmon resonance maximum at about 700 nm. These spectral changes can be explained by simulations of the electromagnetic properties using the finite element method (FEM), assuming growth in spherical elevations at the tips of the prisms (see Figure 3b). The collapse of the plasmon resonances can be attributed to the strong attenuation of the localized electromagnetic field in the bulge.
These observations confirm the crystallographic model of these particles with a partially defective hexagonal close-packed layer. In the future, these results could help material scientists design more stable silver nanoparticles. In addition, the correlative technique described can be used to clarify the kinetics of nanometer-scale corrosion of other materials.