- Home
- Research
- Spectroscopy and Imaging
- Research results
- Multimodal Nonlinear Microscopy Used in the Therapy Monitoring of Cold Atmospheric Plasma Treatment
Multimodal Nonlinear Microscopy Used in the Therapy Monitoring of Cold Atmospheric Plasma Treatment

14.07.2020
By Tobias Meyer // Hyeonsoo Bae // Sybille Hasse // Jörn Winter // Thomas von Woedtke // Michael Schmitt // Klaus-Dieter Weltmann // Juergen Popp
The work presented here was published in 2019 under the title Multimodal nonlinear microscopy for therapy monitoring of cold atmospheric plasma treatment.[1] Improper wound healing affects millions of people in the western world. Cold atmospheric plasma (CAP) is one possible treatment, which is even effective in the case of multi drug-resistant bacteria. The ever-increasing abundance of multi-resistant bacteria poses a major public health challenge in developed countries, especially when considering the aging population. There is, therefore, a great need for such alternative therapies like CAP, especially in the treatment of chronic wounds. To date, the direct application of CAP to or within the human body for therapeutic purposes has been shown to be very effective in the treatment of chronic and inflamed wounds, including wounds infected with multi-resistant microbes; however, the mechanisms behind CAP are not fully understood. In addition, tools for monitoring this treatment are missing (e.g., those used to see whether the area has already been sufficiently treated). The multimodal nonlinear imaging approach combining coherent anti-Stokes Raman scattering (CARS), two-photon excited fluorescence (TPEF), and second harmonic generation (SHG), which has been researched for many years at Leibniz IPHT, has been complemented by two-photon excited fluorescence lifetime imaging (FLIM) for the label-free monitoring of CAP treatment efficiency on human skin and mucosa samples. The influence of the plasma effects has been evaluated by varying the plasma parameters (e.g., duration of treatment, gas composition, and plasma source). CARS and SHG prove the integrity of the tissue structure and visualize tissue morphology and composition, while TPEF and FLIM have been used for treatment monitoring by observing an increase in the overall autofluorescence. By correlating with histochemical staining and FLIM, a highly localized increase in fluorescence was able to be assigned to melanin.
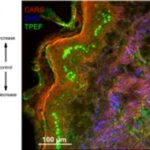
The basic idea of this research project is depicted in Figure 1. Infected wounds are treated with CAP, which results in the inactivation of bacteria by mechanisms which need to be further investigated and better understood. To achieve this goal, multimodal nonlinear microscopy is applied to the treatment area to search for biomarkers for monitoring CAP treatment. In order to search for biomarkers for monitoring CAP treatment, multimodal nonlinear imaging combining CARS, SHG, and 2P-FLIM has been applied to investigate thin tissue sections of skin and mucosa. Different experimental conditions for CAP treatment have been analyzed as shown in Figure 2 A, varying the following parameters: treatment time, plasma source, gas composition, specimen. The sections were analyzed before and after CAP treatment to search for spectroscopic changes related to CAP treatment. For all parameters, a significant increase in autofluorescence was observed after treatment (see Figure 2 A). In Figure 2 B a human skin tissue sample was treated with the kINPen MED® device with an Ar-oxygen gas mixture for 20 s. The increase in autofluorescence is mainly localized in the proliferating and metabolically active basal cell layer (i.e., the melanocytes inside the stratum basale). These cells are also rich in melanin as evident by the comparison with AgNO3 staining for melanin proving localization of the CAP-induced increase in autofluorescence within the melanin layer (see Figure 2 C).
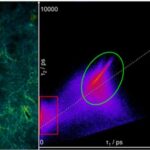
To further analyze the origin of the autofluorescence increase, two-photon FLIM has been employed. In order to prove that the observed strong fluorescence within the epithelium is due to melanin, melanin was selectively excited using the pump laser at 670 nm only, which fits best to melanin two-photon absorption. Melanin two-photon absorption is spectrally broad but decreases from 300 nm to 700 nm. The emission peaks from 450 nm to 600 nm, such that the filter used fits to the melanin emission range. The fluorescence lifetime of melanin has been reported to be in the range of 100‑150 ps. In Figure 3 A, the short lifetime component of two-photon FLIM of the human skin tissue section excited at 670 nm is displayed. Excitation at 670 nm results in bright emission from the basal cell layer characterized by a first lifetime component of 50 ps, which is much lower than the lifetime of free NAD(P)H of 400 ps, but characteristic for melanin. The 2D histogram of the lifetimes t1 and t2 in Figure 3 B reveals two clusters of pixels marked in red and green. The red cluster corresponds to melanin (see top left image). The green cluster represents fluorescence from the other tissue areas.
In conclusion, it has been demonstrated that multimodal nonlinear microscopy combining SHG, CARS, TPEF, and 2P-FLIM is a powerful diagnostic tool used to monitor and investigate the mechanisms of CAP treatment on a molecular level without using exogenous labels. By using specific excitation and emission wavelengths adapted to autofluorescent marker molecules, we believe that the signals which correlate with CAP treatment can be significantly increased and that characteristic lifetime changes can be observed and correlated to specific molecular markers. 2P-FLIM is a highly valuable tool used to investigate the generation of reactive oxygen species (ROS) induced by CAP treatment, which modify the endogenous fluorescence (e.g., by activating emission-free de-excitation pathways).