- Home
- Research
- Spectroscopy and Imaging
- Research results
- Fundamental SERS Investigation of the Interaction between Small Molecules and Silver Nanoparticles
Fundamental SERS Investigation of the Interaction between Small Molecules and Silver Nanoparticles
23.04.2018
In order to study the interaction between low molecular weight substances and different silver nanoparticles, Raman and SERS investigations were conducted using pyridine and selected derivatives. This study contributes to the identification of suitable marker molecules for SERS labels because the spectral signature strongly depends on the orientation of the molecules to the metal surface.
By: Anna Mühlig // Dana Cialla-May // Jürgen Popp
In the past, pyridine has been the focus of a large number of vibrational spectroscopic studies, in particular signal amplification studies on roughened metal electrodes. This paved the way for describing the effect of surface-enhanced Raman spectroscopy (SERS). We have investigated the interaction of pyridine and its derivatives 2-, 3-, and 4-pyridinemethanol (pymeth) and 2-, 3-, and 4-picolylamine (picam) with a citrate or hydroxylamine-reduced silver colloid (c-AgNPs or h-AgNPs) in order to gain insight into the preferred orientation of the molecules to the metal surface. The influence of the substitution position, functional group, and surface properties was taken into account. These factors can have a strong influence on the spectral signature since the intensity of the Raman modes strongly depends on the orientation of the molecules to the metal surface. The results of Raman and SERS spectroscopic investigations were verified by quantum mechanical calculations. This enabled an exact band assignment to be made, which made it possible to obtain clear information on the orientation of the molecules. The key findings of the study are briefly summarized below.
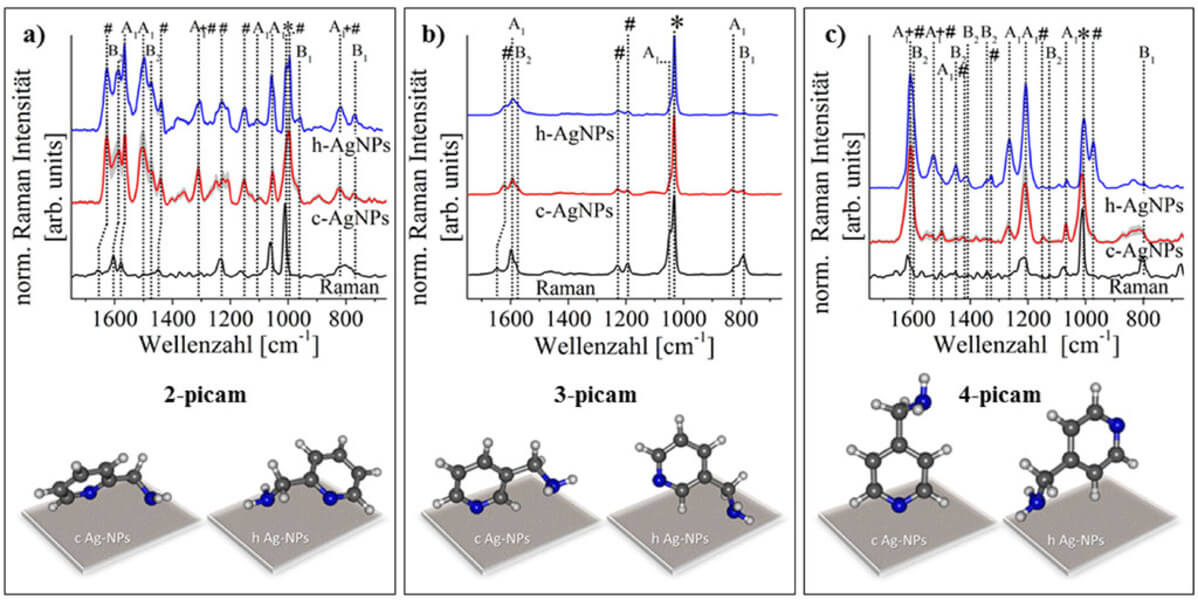
The SERS spectra of 4-picam vary greatly depending on which silver colloid is used. This is due to the fact that the coordination with the metal surface takes place via the nitrogen atom in the ring (with c-AgNPs) or via the functional group in the para position (with h-AgNPS). In the case of 3-picam, the differences in the SERS spectra are smaller since the interaction with both heteroatoms and functional groups at the different silver surfaces is sterically less impeded. Consequently, there are no differences in the SERS spectra of 2-picam because the preferred interaction with different metal surfaces via different functional groups leads to the same orientation relative to the metal surface. As a result, the spectral differences become smaller the closer the two nitrogen atoms of the functional group and the heteroaromatic ring are to each other. These relationships are illustrated in Figure 1.
Furthermore, the influence of the type and position of the functional group was examined. For this purpose, SERS spectra of 2-, 3- and 4-pymeth and 2-, 3- and 4-picam were recorded using citrate-reduced silver nanoparticles (c-AgNPs). These interact preferably via the heteroaromate nitrogen atom with the analyte molecules. Since the functional group is best able to interact with the metal surface in the case of ortho substitution for a preferred coordination via the ring nitrogen atom, these investigations reveal the greatest differences in the SERS spectra of 2-picam and 2-pymeth. This is due to the fact that the functional group is very close to the metal surface and can thus contribute significantly to the spectrum. The differences in the SERS spectra become smaller as the functional group moves further away from the metal surface (i.e., for meta- and para-substituted functional groups). In summary, the SERS spectra are influenced by various parameters. The corresponding SERS spectra are shown in Figure 2.
The study presented here shows that the type of silver nanoparticles, as well as the type and substitution position of the functional group, can change the SERS spectrum. These findings are of particular importance for the use of marker molecules in SERS experiments. In this way, spectral positions can be highlighted that do not overlap with other Raman modes (e.g., the background) and thus permit the correct identification of the marker molecule.
Funded by: BMBF