- Home
- Research
- Quantum Detection
- Research results
- Nanochemical imaging for supporting theranostics
Nanochemical imaging for supporting theranostics
28.03.2019
Photo-induced force microscopy complements established spectroscopy techniques
By D. Täuber
Targeting drugs to cancer cells and cellular infection sites has become a very successful and still further promising road in the combat against cancer and systemic infection, as the tailored release allows for enhanced concentration of the drug at targeted sites while the chemical pressure for the organism in total is kept low. The successful development of targeted drugs relies on careful evaluation of the response of addressed sites to the intended treatment.
The recently developed Photo-induced Force Microscopy (PiFM) enables nanochemical imaging of surfaces by combining excitation with IR light and detection of the induced electro-magnetic near-field via force microscopy equipped with a conductive tip. In contrast to optical detection methods, the new label-free approach is independent of optical scattering and thus allows for enhanced signal resolution while it also prospers from high spatial resolution down to a few nanometers. These features render PiFM promising for complementing established spectroscopy techniques.
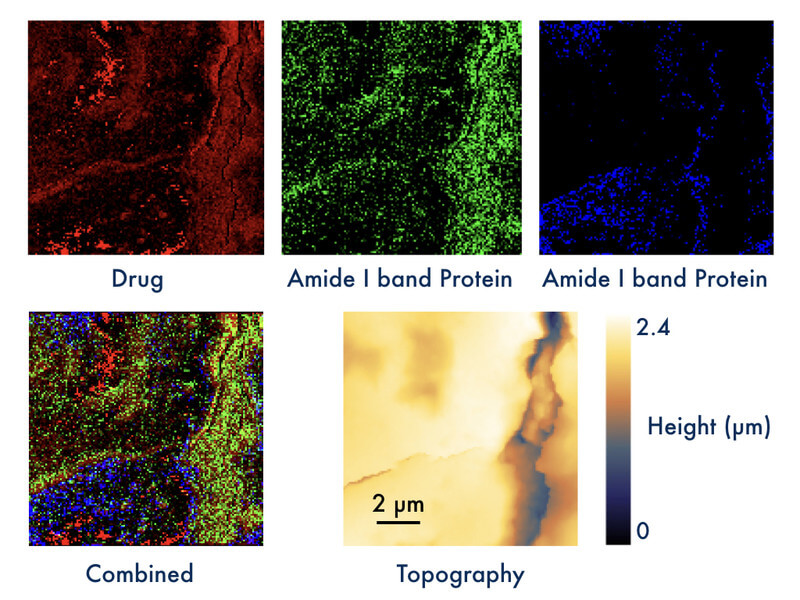
Dr. Daniela Täuber, head of the group Biopolarisation in the Department of Quantum Detection demonstrated the power of the new method by visualizing local distributions of a drug in mouse liver tissue (see Figure 1). Dr. Anuradha Ramoji from the Department of Clinical Spectroscopy and Diagnostics at Leibniz-IPHT had provided an IR spectrum of the pure drug, and the contrast in the top left image (red) was obtained at an IR absorption peak of the drug. Taken at a spatial resolution slightly below 100 nm, high PiFM signal (bright) was obtained at several locations in the scan. PiFM spectra recorded at such positions show characteristics similar to the IR spectrum of the drug. Spectra are not shown here, as they are content of a planned publication. The sample was further characterized by the response at two frequencies related to amide bands of proteins (green and blue). The spatial resolution is already more than one magnitude higher than the range reached by IR spectroscopy imaging, and it can be further increased up to a few nm. Samples were provided by their collaborator Dr. Adrian Press, who heads the Nanophysiology group in the Anesthesiology and Intensive Care Medicine Department of Prof. Michael Bauer at Jena University Hospital. The PiFM measurements were conducted at a cooperating facility in collaboration with Dr. Ramoji and Kay-Jovanna Benecke from Dr. Press’s group.
Funding for an instrument for PiFM at the Leibniz-IPHT will be provided by the TAB in 2019 for „the development of novel technologies for the detection of pathologic variations and localized visualization of the response to therapy using photonically polarizable biological species and photo-induced force microscopy,“ which was initiated by Prof. Heidemarie Schmidt, head of the Department of Quantum Detection.
Due to the novelty of PiFM, analysis methods still have to be developed, which requires investigating less complex samples. Thus, as a next step, Dr. Täuber shaped a project for investigating nanoparticles and cell cultures using PiFM and comparing the results with well-established IR- and Raman spectroscopy in collaboration with Prof. Ute Neugebauer and Dr. Ramoji from the Department of Clinical Spectroscopy and Diagnostics, and Prof. Felix Schacher and Philip Biehl from the Jenano Group at Friedrich-Schiller University Jena. Two master students (Nila Krishnakumar, Master of Photonics, Abbe School of Photonics, FSU Jena, and Anika Strecker, Master of Medical Engineering, Ernst-Abbe University of Applied Sciences Jena) started working on this topic in November 2018.
Funded by: TAB
