- Home
- Research
- Functional Interfaces
- Research results
- Nanostructured: Shaping the Energy and Water of the Future
Nanostructured: Shaping the Energy and Water of the Future
25.08.2023
In recent years, hydrogen has become more and more the focus of energy policy discussions, and promises to play an important role in energy supply in the future. At Leibniz IPHT, scientists are researching how hydrogen can be successfully obtained by light-driven water splitting as part of an internal innovation project.
At Leibniz IPHT, silicon-based nanostructures and their interaction with light have been researched for many years. Such semiconductor structures on the nanometer scale are of interest, for example, as components for solar cells in photovoltaics, as biocompatible and innovative structures in cancer theranostics, or as self-cleaning surfaces in materials science.
Semiconductor materials have also proven to be valuable key technologies for the splitting of water into its components, hydrogen and oxygen, using light, the so-called photocatalytic water splitting. Researchers around the globe are working to further improve the production of hydrogen by separating water by experimenting with different catalyst materials.
Silicon nanostructures represent a promising option. In order to develop their potential for photocatalytic water splitting as well as to identify ways for efficient hydrogen production, Leibniz IPHT scientists from the Research Departments Functional Interfaces, Nanobiophotonics and Nanooptics investigated silicon nanostructures under laboratory conditions.
For this purpose, ultrafine nanowires of less than 100 nanometers in size were structured from a silicon layer, or on a wafer using “top-down” wet chemical etching processes and decorated with silver nanoparticles. By means of this structuring and refinement method, the chemical-physical properties of the silicon can be specifically modified. The thus created silicon structures refined with metallic nanoparticles, were then brought into contact with an aqueous solution and artificial light.
In scientific investigations using microscopic and spectroscopic methods, the researchers found that the silicon nanowires coupled with silver nanoparticles were able to significantly increase the efficiency of hydrogen production. Thanks to the silicon nanostructures functionalized with silver, the amount of hydrogen produced was significantly increased during the chemical processes and the simultaneous formation of silicon suboxides on their surface. The rate of hydrogen generation produced in this way is at least five times higher than the values described in the literature, and thus comparable to hydrogen generation based on titanium oxide, a semiconductor which has been known as a catalyst material for many years.
The detailed understanding of the nanomaterial surface down to the atomic level gained during the project, the interaction processes at its surface during the chemical reaction, and the ongoing photocatalytic processes provide an important contribution to further research into effective hydrogen production. The hydrogen produced basing on these principles could be stored, used for energy generation, but also for water purification.
The innovation project laid the foundation for subsequent projects funded by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) and the Central Innovation Programme for small and medium-sized enterprises (Zentrales Innovationsprogramm Mittelstand, ZIM) of the German Federal Ministry for Economic Affairs and Climate Action (Bundesministerium für Wirtschaft und Klimaschutz, BMWK).
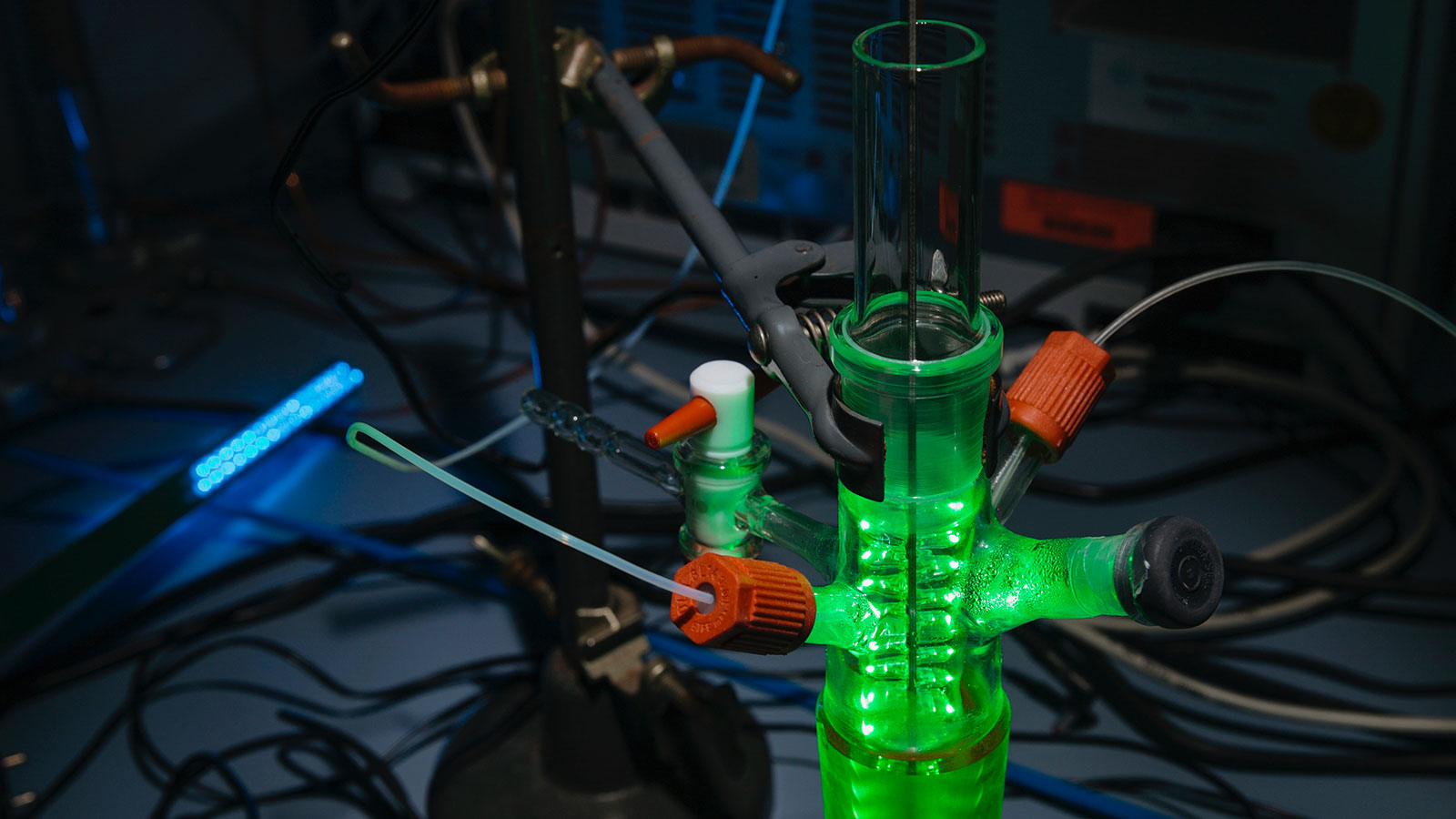
In the picture:
In laboratory experiments, scientists at Leibniz IPHT were able to generate hydrogen through
refined silicon nanostructures.
©Sven Döring
