- Home
- Technology Groups
- Competence Center for Micro- and Nanotechnologies
- Research results
- Microfluidics for Nanoparticle Synthesis and LOC Technology
Microfluidics for Nanoparticle Synthesis and LOC Technology
23.04.2018
The application of microfluidic synthesis principles for the production of plasmonic nanoparticles opens up new possibilities for the production of form-anisotropic nanoparticles with controlled geometry and narrow size distribution. Microfluidic techniques made it possible to separate the nucleation and growth processes which run in parallel in conventional methods and to optimize them separately. With the knowledge gained in this way, both shape and size can be precisely determined, which opens up efficient access to nanoparticles with properties that are optimal for sensory applications.
By: Matthias Thiele // Andrea Csaki // Thomas Henkel // Wolfgang Fritzsche
As optically readable nanosensors, plasmonic nanoparticles are becoming increasingly important in application fields such as analytics and diagnostics. Color, spectroscopic properties, and the sensory function can be controlled by the size, shape, and molecular architecture of the nanoparticles. Sensory nanoparticles with different localized surface plasmon resonance (LSPR) can be read out in parallel. Multiplexing and the parallel readout of several analytical parameters can thus be achieved using a mixture of nanoparticles with different spectral and sensory properties both at the level of the individual nanoparticle and by parallel readout of a particle collection. As the optical and sensory properties of the nanoparticle collections used increase in uniformity, the sensitivity and precision of these LSPR-based assays increase. This results in the need to develop and implement new and efficient methods for the specific and reproducible synthesis of plasmonic nanoparticles with monodisperse size distribution and uniform geometry.
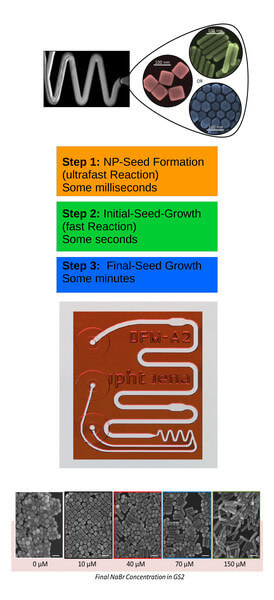
The chemical synthesis of form-anisotropic plasmonic nanoparticles, such as that of gold nanocubes, is a multi-stage synthesis in which several reaction steps are carried out sequentially or in parallel. In conventional batch syntheses, these three steps are carried out competitively. For optimal control of the synthesis and thus of the properties of the nanoparticles obtained, it makes sense to separate the individual steps. This enables each of the sub-processes to be carried out under optimal conditions – partly using different reagents and concentrations – and to be investigated separately. This is achieved with the microfluidic chip systems developed by the microfluidics research group.
The following sub-processes are important for the synthesis of plasmonic nanoparticles: nucleation, initial growth, and subsequent growth of the nanoparticles. These sub-processes differ significantly in their reaction speed. Nucleation, as an extremely fast reaction, is completed after only 20-100 milliseconds. The initial growth then takes place within a few seconds while reaction times of several minutes are required for the final growth step.
For the preparation of plasmonic nanoparticles with uniform properties, all reagents used must be present in the correct concentration, avoiding local concentration differences. Therefore, mixing the starting materials must be completed before the actual chemical reaction starts.
The most critical step for rapid mixing is the nucleation reaction. By using a Dean-flow mixer, mixing can be achieved within less than one thousandth of a second at flow rates of approx. 60 µl/second. As a result, seed nanoparticles at a size of approx. 2 nm are obtained. The subsequent initial growth step requires mixing within less than one second at low flow rates. For this purpose, a multi-lamination mixer (which operates based on the rotate-split-and-recombine (ROSAR) algorithm developed at IPHT) is used at flow rates in the range of 1 µl/second. It works at low Reynolds numbers of < 1 and can, therefore, also be used for the final reaction step.
Based on this architecture, the nanobiophotonics research group succeeded in completely separating the reaction steps and their individual optimization and research. The results were published in the Lab on a Chip magazine [Thiele 2017]. For the first time, it has been demonstrated that the shape of the nanoparticles formed in the final growth step depends to a large extent on the nature of the halide ions present in the reaction solution. By selecting the appropriate halide ion (e.g., chloride, bromide, or iodide) and its concentration, the geometry of the resulting nanoparticles (i.e., cubes, spheres, or rods) is precisely predetermined. Based on this knowledge, the shape and size of plasmonic nanoparticles can be precisely adjusted and novel particle types (e.g., gold nanocubes) can be synthesized with excellent uniformity in shape and size and made available for research in the field of nanobiophotonics.
The use of microfluidic components also offers many advantages in the field of diagnostics. In this way, molecular biological reactions such as DNA amplification PCR [Singh] and droplet-based SERS detection of disease biomarkers [Hassoun] can be carried out in a more defined manner compared to conventional methods.
Funded by: EU
