- Home
- Research
- Microscopy
- Research results
- Better than a lens – A novel concept to overcome the SNR-limit given by the Fermat principle.
Better than a lens – A novel concept to overcome the SNR-limit given by the Fermat principle.
28.03.2019
By dividing the pupil of an incoherent imaging system, it is possible to improve the signal-to-noise ratio (SNR) in the imaging of high-frequency object structures. This is achieved by limiting the number of photons to a given number and thus represents a novel possibility of SNR enhancement. Applications lie in biology and industrial manufacturing.
By J. Becker // R. Förster // R. Heintzmann
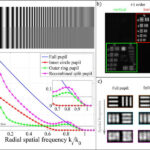
In the practical application of fluorescence microscopy, the problem often arises that biological structures can no longer be represented with sufficient contrast due to the poor SNR. One reason for this is the information transfer of the optical system, which is getting worse in the direction of high-frequency object structures (see optical transfer function (OTF) in Fig. 1a). On the other hand, even when using an ideal detector (completely noise-free), photon-limited noise can still be expected, which leads to a constant frequency spectrum. This results in an effective loss of resolution of the imaging system, since signal and noise cannot be distinguished from each other above a certain frequency structure (Fig. 1a).
Typically, this problem is solved by sequential acquisition of several images and subsequent averaging, but comes at the cost of loss of temporal resolution. Another possibility would be a stronger illumination, whereupon the sample to be investigated emits a larger number of photons and thus increases the SNR. However, since this also leads to an increase in the light exposure within the biological sample, this method does not seem to be a viable solution for all applications. From a theoretical point of view, the question arises whether it is possible to obtain an SNR improvement, provided that only a given number of photons can be detected. Due to latter assumption, both methods presented above do not represent an alternative, since in both cases a larger amount of light is required.
The underlying idea is to separate the pupil of the optical system. Thus, in principle, two single images are obtained (Fig. 1b), which together detect the same number of photons of the total pupil separated from each other. This splitting reduces the signal and the noise in the individual sub-images, since the strength of the noise depends on the number of photons (photon-limited noise). However, a closer look shows that in one of the sub-images the signal of high-frequency structures is imaged with the same strength as without the pupil division. By clever calculation, it is then possible to generate an image that shows an increased SNR of the high-frequency object structures for the same number of photons. This is shown in practice by an improved contrast and finally by an increase of the effective resolution limit of the system. As can be seen from Fig. 1a), the method leads to an improvement in the imaging of fine details (“better than a lens”), but also to a poorer imaging of coarse structures, which are generally easier to measure.
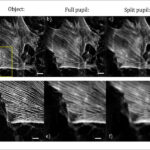
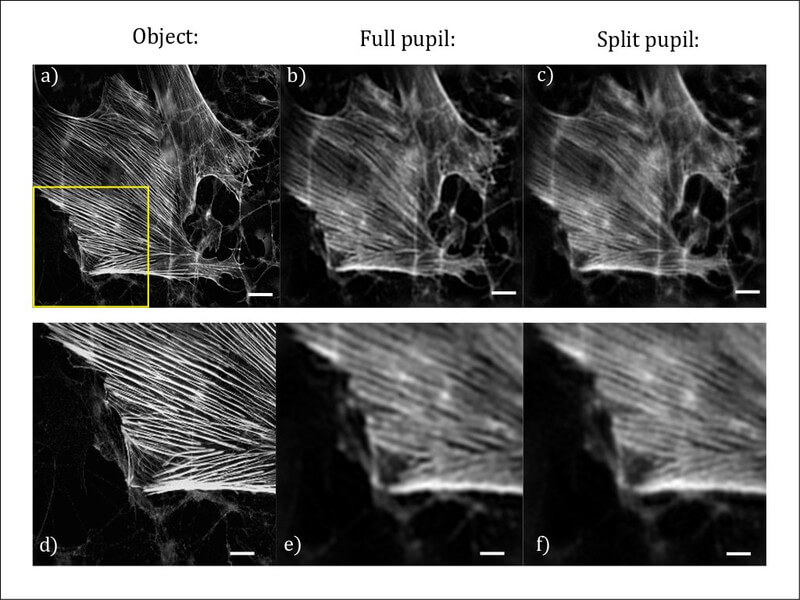
A simple realization of this “split pupil imaging” concept was tested by using a 4f imaging system with a spatial light modulator (SLM) in the pupil plane. Two orthogonally oriented phase grids, which correspond to the spatial distribution of the sub-pupils, generate the two required sub-images on a single camera (Fig. 1b). After the corresponding computer-based processing of the raw data, the expected improvement of the contrast of the imaged line structures could be confirmed experimentally (Fig. 1c).
Potential fields of application of the presented method lie mainly in the imaging of high-frequency structures as they occur in (cell) biology (Fig. 2) or industrial production monitoring. For the former, the concept presented is currently being implemented using a commercial microscope stand. The pupil splitting will be done by a specially manufactured mirror, which will considerably increase the efficiency of the optical system. This offers the possibility to use the SNR improvement also for a microscope with high numerical aperture, thus higher resolution limit. This opens up a broad field of important applications in basic biological research.
Funded by: EU, Free State Thüringen