- Home
- Technology Groups
- Competence Center for Micro- and Nanotechnologies
- Research results
- On the Trail of the Virus
On the Trail of the Virus

12.05.2021
SARS-CoV-2 or influenza? Researchers are developing an optical method to quickly identify viruses. To do this, they combine topographic and spectroscopic methods
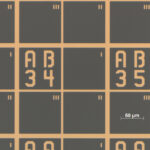
AA 33, AA 34, AA 35 horizontally; AB 33, AC33, AD 33 vertically… The 50-micrometer tiny golden numbers on the fine microscope cover sheet are not visible to the naked eye. The coordinate system on the chip is reminiscent of the game “sink ships” – except that the researchers are using it to detect and localize virus particles rather than boats. In order to characterize SARS-CoV-2 quickly and in a structure-specific manner, an interdepartmental team at Leibniz IPHT is combining topographic and spectroscopic methods. In this way, the researchers want to develop a method to quickly and reliably identify individual virus particles in clinical sample material using Raman spectroscopy. Whether a person is infected with influenza or with SARS-CoV-2 could thus be distinguished within minutes.
The basis for the research is the chip with the box pattern. It goes back to an idea from the group of nano-optics expert Jer-Shing Huang. Huang and his team use it to localize nanoparticles and to measure or process them in a targeted manner. The ultra-thin chips are manufactured using mask-based photolithography in the institute’s clean room. Using vacuum processes, the employees vaporize the fine structures with gold. After a final “lift-off,” only the box pattern and the lettering remain as gold structures on the chip.
Working with the filigree materials requires a delicate touch. “For optical and metrological reasons, we use very thin glass substrates; these are microscope cover sheets that are just 170µm thick,” explains Uwe Hübner, head of the Competence Center for Micro and Nanotechnologies. “They tend to break during the processing steps – so for one chip you have exactly one attempt.”
The chips are then first used under the scanning probe microscope. The team of nanoscopy expert Volker Deckert uses them to examine samples of SARS-CoV-2 virions; these are individual virus particles outside a cell. The research partners from the working group of virologist Stefanie Deinhardt-Emmer from the Institute of Medical Microbiology at Jena University Hospital have previously labeled the viral RNA with the dye SYBR gold, which does not change the size of the virions.
Three methods, one statement
Deckert and his team now first examine the samples topographically, i. e. make a quick preselection of potential virus candidates for further examination according to morphological criteria based on size. Without the specially manufactured substrates, says Volker Deckert, the virus particles, which are between 50 and 100 nanometers in size, would not even be detected in the subsequent experiments. Using the grid, however, the particles in question can be precisely assigned by their location – and the sample goes to the next round: to Christian Eggeling in his “Biophysical Imaging” research department.
Eggeling and his team examine the sample at the same location with the fluorescence microscope. Using the fluorescence of the SYBR gold dye, they find the labeled RNA of the virions. The substrate is returned to Volker Deckert for inspection: he uses atomic force microscopy to check again whether there are any losses or changes in the sample. In the vast majority of cases, however, these are negligible, says Deckert. Taken together, the fluorescence images and the topographies now provide a reliable identification of the virus particles. To do this, the images are correlated with each other, i. e. superimposed.
To find out whether the virus is active or whether RNA has been lost, another method now comes into play: Using super-resolution fluorescence microscopy, Rainer Heintzmann and his team from the “Microscopy” research department focus on the spike protein of the virus. This protein is used by SARS-CoV-2 to dock onto cells. Via the spike-like structures, the virus binds to specific receptors on the surface of human cells in order to infect them.
Because the RNA dye is not suitable for super-resolution, Heintzmann and his team label the sample with antibodies. These can recognize the virus on the basis of the spike protein. Using super-resolution fluorescence microscopy, the researchers achieve a resolution of about 50 nanometers. This allows them to identify the spike protein of the samples. However, the labeled samples can no longer be used for further investigations afterwards.
Three methods, one statement: via the precise local comparison, the researchers succeed in reliably narrowing down the size distribution of SARS-CoV-2 virions in the sample. “It is an essential step to unambiguously identify SARS-CoV-2 particles and to quickly pre-select potential candidates for further investigations without labeling,” explains Volker Deckert: “based solely on morphological properties and under clinically relevant environmental conditions.”
This pre-selection finally allows Deckert and his team to investigate the structure of the identified viral particles at the nanoscale using tip-enhanced Raman spectroscopy (TERS). “With TERS, we can look specifically at individual virus particles and measure them precisely with nanometer resolution and chemical specificity,” Deckert said. “Using the TERS spectra obtained, we can capture the sample surface of the particles very quickly; in less than a second per spectrum.”
The goal: label-free rapid diagnostics
Fast, accurate, and label-free, studying SARS-CoV-2 by tip-enhanced Raman spectroscopy could, in perspective, allow us to learn more about the mechanisms by which the virus interacts with the cell surface. “Studying this is only possible if dyes are not used,” Deckert says.
For rapid diagnostics, the combined method could open up entirely new possibilities. Topographic measurement could be used to determine very quickly whether or not virus particles are present in a sample. The researchers are now working on optimizing the method so that they can detect whether a sample contains SARS-CoV-2 or influenza viruses within a very short time. According to Volker Deckert, this would even be possible before antibodies are present.