- Home
- Research
- Clinical Spectroscopic Diagnostics
- Research results
- Spectroscopic Characterization of Innovative, Light-activatable Complexes for the Intracellular Administration of Carbon Monoxide
Spectroscopic Characterization of Innovative, Light-activatable Complexes for the Intracellular Administration of Carbon Monoxide
28.03.2019
By R. Mede // P. Hoffmann // C. Neumann // H. Görls // M. Schmitt // J. Popp // U. Neugebauer, // M. Westerhausen
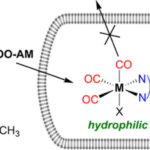
Carbon monoxide (CO) is known as a highly toxic gas; however, in low concentrations, it also shows a multitude of positive physiological effects. For the controlled application of CO directly in eukaryotic cells, CO must be supplied in a targeted and safe manner. This can possibly be achieved with the help of CO-releasing molecules (CORMs). For CORMs, there are various triggers for the release of carbon monoxide. If the release of carbon monoxide is triggered, for example, by light, then they are referred to as PhotoCORMs. As part of the DFG research project “Heme and heme degradation products” some promising compounds were designed and synthesized by project partners from the Institute of Inorganic and Analytical Chemistry of the Friedrich Schiller University Jena. In the current publication, two PhotoCORMs based on the acetoxymethyl (AM) concept were investigated. The metal carbonyl complexes of these PhotoCORMs are lipophilic in nature and equipped with an AM side chain for optimal uptake into the cell. This allows the complex to pass through the cell membrane. Intracellularly, the AM group is cleaved by esterases and the resulting ionic and more hydrophilic product remains trapped within the cell (see Fig. 1).
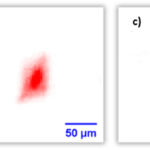
In order to test whether the innovative design of the new PhotoCORMs really leads to an efficient cellular uptake and whether the intracellular release of CO is subsequently possible, cell studies were performed using FT-IR spectroscopic imaging. For both CORMs a noticeable cellular uptake was able to be detected, a uniform intracellular distribution of the CORMs visualized, and afterwards the successful in vitro release of CO shown unequivocally (see Figs. 2 and 3). Especially with regard to the temporally and quantitatively controlled administration of CO, the maximum amount of CO released per CORM and the number of CO released per unit of time must also be determined. The corresponding kinetics of the release of CO were first determined via FT-IR spectroscopy by measuring the amount of CO released in the gas phase above the CORM solutions during irradiation with light of different wavelengths (365 nm, 405 nm, and 470 nm). It was shown that always all three CO ligands of the different AM-CORMs were released into the gas phase and that the PhotoCORMs completely disintegrated when irradiated. In addition, the photoinduced decay reactions were observed directly in the aqueous solutions using time-resolved UV-Vis spectroscopy. For both methods it was shown consistently that the half-life increases with irradiation via light with a higher wavelength and thus the release of CO is slower (470 nm << 405 nm < 365 nm). A comparison of these two spectroscopic methods with regard to kinetics revealed that the decay rate can best be determined directly by the decay of the reactant (UV-Vis spectroscopy) since, when observing product formation in the gas phase above the reaction solution using FT-IR spectroscopy, the diffusion time of the CO is also added. The advantage of the latter method is the noncontact and direct determinability of the CO molecules released per CORM, even under different conditions in the solution. Furthermore, it is essential to determine the identity of the remaining end products in order to exclude possible side effects of these residues in the cell or estimate their extent. The products from the light-induced decay of CORMs were investigated using FT-IR spectroscopy. It was shown that relatively harmless manganese(II) hydrogen phosphate is formed under physiological conditions in phosphate-buffered salt solution.
In the context of physicochemical characterization, we were able to successfully demonstrate using powerful spectroscopic methods that the innovative AM concept applied here allows an extremely efficient intracellular administration of PhotoCORMs and thus paves the way for the further investigation of the effect of CO release. Currently, spectrometric methods are being used to quantify cellular CORM uptake and elucidate the corresponding uptake mechanisms.
Funded by: DFG, BMBF