- Home
- Research
- Nanobiophotonics
- Research results
- Shape-dependent catalytic activity of gold and bimetallic nanoparticles
Shape-dependent catalytic activity of gold and bimetallic nanoparticles
21.12.2022
Plasmonic (noble metal) nanoparticles allow photocatalytic degradation of pollutants. This effect was investigated for the reduction of the model molecule methylene blue with different types of nanoparticles. In particular, formanisotropic and bimetallic particles have proven to be very efficient.
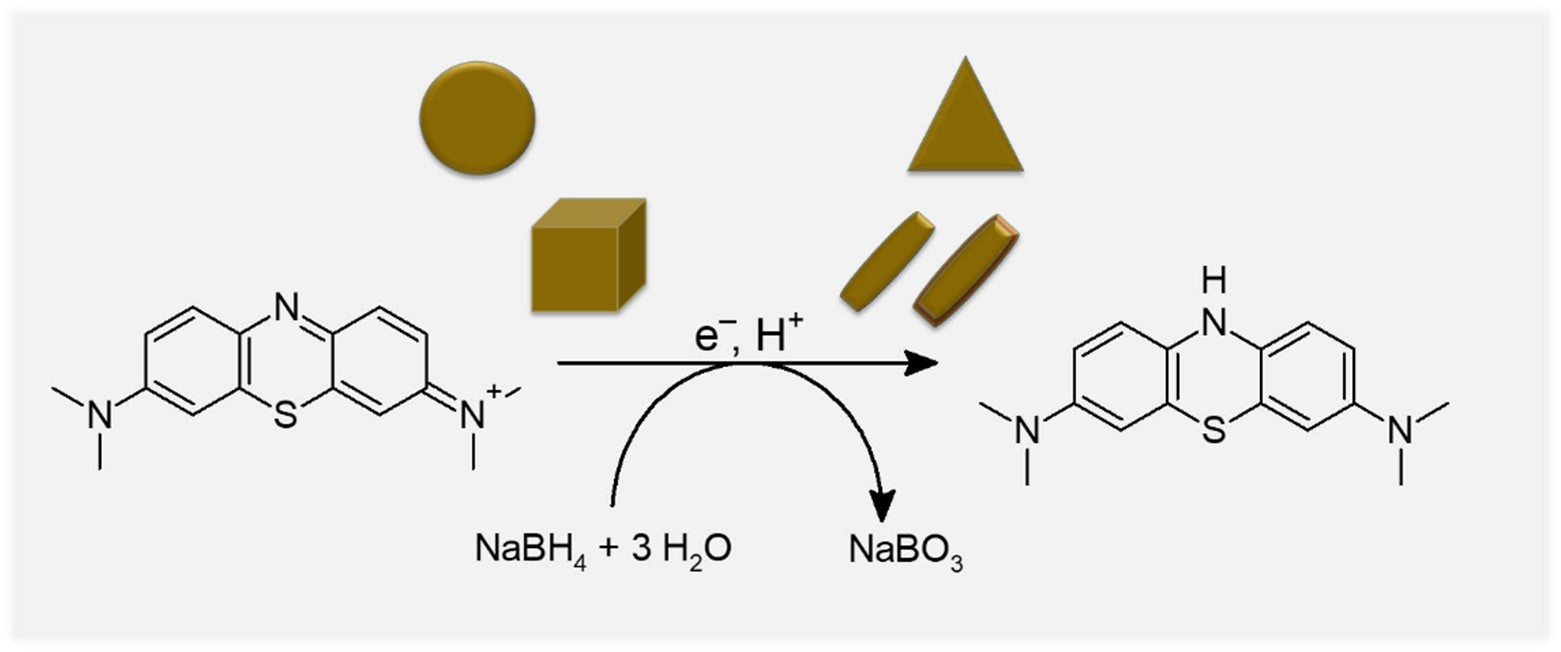
Pollutants of anthropogenic origin can pose a significant threat to our environment and health. Therefore, efficient methods for the targeted degradation of these substances are of high socio-economic relevance. Due to their special optical properties, plasmonic nanoparticles are particularly suitable for such applications. Nanoscale noble metal nanoparticles (e. g. of gold and silver) show a strong absorption band in the visible spectral range of sunlight. This absorption band of the so-called localized surface plasmon resonance (LSPR) is caused by the collective oscillation of the conduction electrons in the nanoscale particles, with corresponding resonance of the incident light. Gold nanospheres exhibit a resonance maximum comparable to the light intensity maximum of sunlight at about 500 nm. The catalytic potential of plasmonic nanoparticles was tested for the reduction of methylene blue to leucomethylene blue in solution. In this process, an electron transfer from the reducing agent sodium borohydride to the methylene blue occurs at the nanoparticle, and the degradation of the methylene blue could be detected spectroscopically. The investigated nanoparticles differed in shape and material composition. Gold spheres, cubes, prisms and rods, as well as rods with palladium and platinum shells were tested here. In comparison with the reference samples without particles, there was generally an increase in turnover, so that a catalytic effect could be demonstrated for all nanoparticles. In particular, the formanisotropic and bimetallic particles showed higher turnover. This was not dependent on the particle area, but primarily on the particle geometry. The more angular and angular a nanoparticle is, the higher its catalysis efficiency.
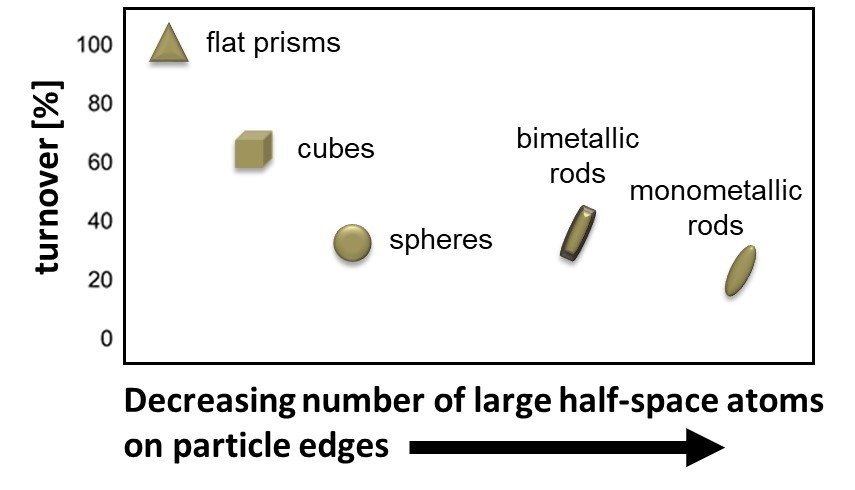
In the picture:
The degradation efficiency is higher the higher the shape anisotropy of the nanoparticles and in comparison, bimetallic nanoparticles also show higher degradation rate compared to the monometallic ones.
Gold nanoprisms with tips and sharp edges showed the highest conversion, followed by cubes and rods. So-called hotspots for the electric field are formed at the corners due to field enhancement. Comparison of gold and bimetallic nanorods showed that the addition of the catalytically active second metal platinum or palladium as sheath material accelerates dye degradation. The rate constants of the degradation reaction were higher than those of the pure gold nanorods. The results provide insight into the catalytic activity of nanoparticles and help in the development of further catalytic applications. The Molecular Plasmonics Work Group has been involved in the design and fabrication of form-anisotropic and bimetallic plasmonic nanoparticles and the investigation of their potential for bioanalytics and catalysis for over 20 years.
Funded by:
Deutsche Forschungsgemeinschaft (DFG)
Project Partners:
TU Ilmenau
In the image above:
Scheme of photocatalytic degradation of the model pollutant methylene blue in the presence of sodium borohydride
