- Home
- Research
- Functional Interfaces
- Research results
- Charge Separation in Functionalized CdSe@CdS Nanorods – Influence of the Size of the Metal Particle
Charge Separation in Functionalized CdSe@CdS Nanorods – Influence of the Size of the Metal Particle
12.04.2018
CdSe@CdS nanorods functionalized with metal nanoparticles are efficient photocatalysts for generating hydrogen from water. The catalytic efficiency depends heavily on the size of the metal nanoparticle, whereby an optimal size exists. With the help of spectroscopic methods, this effect could be attributed to how much the efficiency of charge separation at the semiconductor/metal interface depends on the size.
By: Maria Wächtler
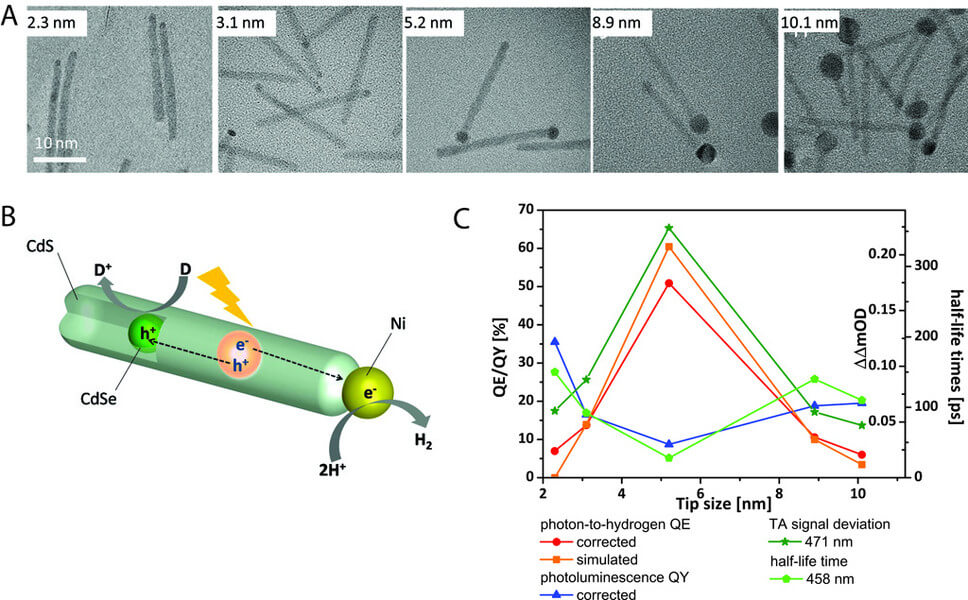
The splitting of water into hydrogen and oxygen driven by sunlight is a sustainable source of clean fuel. CdSe@CdS nanorods functionalized with metal nanoparticles (see Figure 1) are interesting novel photocatalysts for the water-reduction half reaction. They consist of a spherical CdSe core which is embedded in a CdS nanorod. The spatially asymmetrical semiconductor nanorods can be selectively functionalized at one end with a metal nanoparticle (e.g., Pt or Ni). In these structures, the CdSe@CdS nanorod acts as a light-absorbing unit, while the catalytic reaction takes place on the surface of the metal nanoparticle. Light absorption creates an electron-hole pair which then dissociates. The hole localizes in the CdSe core, while the electron is transferred to the metal nanoparticle, where it is then available for the reduction of water to form hydrogen. The remaining hole must be removed by an electron donor to completely regenerate the catalyst. Currently, sacrificial electron donors such as isopropanol are used for this.
On the one hand, efficiency optimization of the conversion of photons into hydrogen in these structures can be achieved by modifying the composition and dimensions of the semiconductor structure (e.g., core size and nanorod length). The influence of these parameters on efficiency has already been extensively investigated in recent years and is widely understood. On the other hand, the influence of the properties of the metal nanoparticle has been insufficiently characterized thus far, especially with regard to its composition and size.
In cooperation with colleagues from Israel, we carried out a study to investigate the influence of the size of the metal nanoparticle on catalytic efficiency. The optimum size of the metal nanoparticle was found to be 5.2 nm for a series of CdSe@CdS nanorods functionalized with Ni nanoparticles with varying diameters between 2.3 nm and 10.1 nm (see Figure 1). In general, the catalytic efficiency of the system can be determined by two potentially size-dependent processes: 1) charge separation at the semiconductor/metal interface and 2) the activity of the nanoparticle surface for the catalytic reaction. In order to gain better insight into the cause of the observed size effect, the charge separation process at the semiconductor/metal interface, depending on the size of the metal nanoparticle, was investigated using (time-resolved) spectroscopic methods. The minimum quantum yield of photoluminescence correlates with the minimum half-life of the electrons in the conduction band and the maximum yield of the generated charge-separated state determined by time-resolved transient absorption spectroscopy (see Figure 1). The photoluminescence quantum yield and half-life of the electrons in the conduction band are reduced by the electron transfer to the Ni particle. The maxima/minima, in turn, correlate with the maximum of photocatalytic efficiency. This suggests that the observed size effect in catalytic efficiency is primarily determined by the charge separation process at the semiconductor/metal interface.
The existence of the optimal size of the metal nanoparticle, for which a maximum efficiency in charge separation is observed, can be explained by the superposition of two effects with opposed size dependence. These effects affect the height of the energy barrier that must be overcome during electron transfer and thus determine how efficiently the charge separation step can compete with relaxation processes of the exciton formed by light absorption. On the one hand, there is a Coulomb blockade, which is particularly pronounced in the smallest nanoparticles and counteracts the transfer of an electron to the metal particle. On the other hand, there is a size-dependent Schottky barrier at the CdS/Ni interface, which increases with increasing size. The observation of an optimal size of the metal domain for charge separation at the semiconductor/metal interface is of far more importance than photocatalysis applications, and it influences all areas in which charge separation processes play a role at nanoscale semiconductor/metal interfaces.
Funded by: EU, FCI
